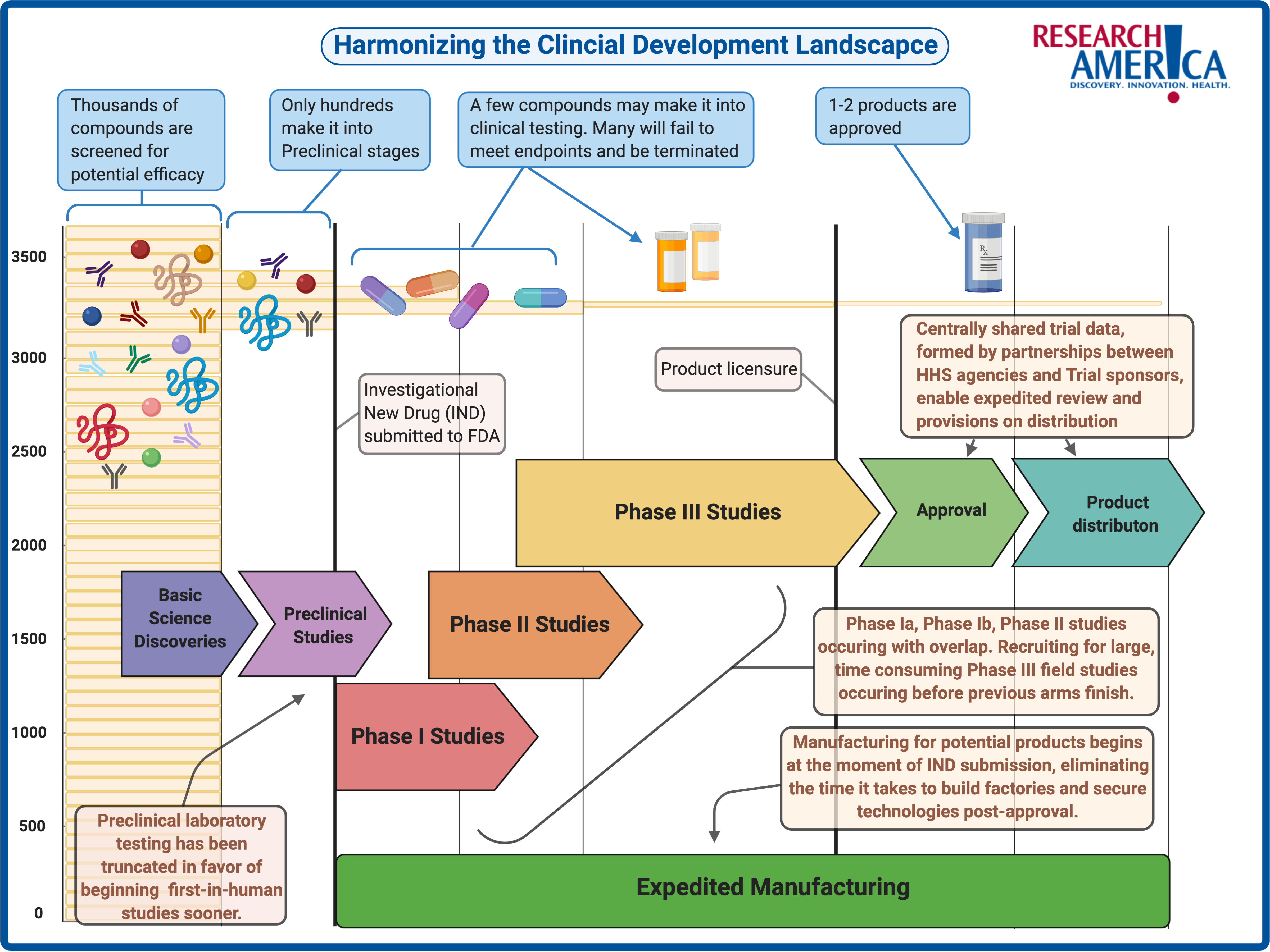
How Does a Vaccine Get Tested and Produced?


Much like drugs, vaccine candidates that seem promising during laboratory research are assessed and validated based on their performance in clinical trials. In the U.S., making it to this step requires a trial sponsor to submit an Investigational New Drug (IND) application to the FDA for review.6 This application most commonly highlights technical data on immunogenicity — the ability to elicit a targeted immune response —, the mechanism of action from animal testing, and importantly, the resources needed for scaling up production.6
In a recent vaccine-development-focused installment of the popular American Public Health Association and National Academy of Medicine’s webinar series, COVID-19 Conversations, Dr. Paul Offit, Director of the Vaccine Education Center and attending physician at the Children’s Hospital of Philadelphia (also a co-recipient of the Research!America 2020 Geoffrey Beene Foundation Builders of Science Award) highlighted that in the era of COVID-19, stages such as animal testing have been “completely truncated” for speedier outcomes. Vaccine trials historically require more time, because unlike drugs, successful vaccines are administered to healthy individuals rather than ill patients, meaning that the safety and effectiveness profiles are under more scrutiny than traditional drug trials.7
The first in-human process begins with phase I studies. These trials are usually small, not randomized, and require volunteers with healthy immune systems that have no previous exposure to the germ that the vaccine aims to protect against.7 The recruitment of these volunteers is a pivotal first step in assessing the vaccine’s safety profile. Next, either additional phase I studies or the beginning of phase II studies are conducted that examine the vaccine’s performance in other small populations and those previously infected by the germ. These populations are more representative of target demographics for post-approval administration and include constraints such as age and potential co-morbidities.7 Sometimes, phase II studies are conducted in a manner known as a challenge study. This involves vaccinating healthy participants, allowing them to develop antibodies against the germ in question, and then presenting the germ to them to observe the participants’ ability to ward off infection.7 For numerous reasons, this is not always a suitable testing approach. Dr. Kathleen Neuzil, Director of the Center for Vaccine Development and Global Health at the University of Maryland School of Medicine, mentioned in the COVID-19 Conversations vaccine webinar that this is the case with COVID-19. She added that because viable treatments are not yet on the market and because SARS-CoV2 is particularly dangerous for clinicians to handle, challenge studies are not currently suitable.
While phase I and phase II studies are vital for the progression of a vaccine’s likelihood of approval, Dr. Offit aptly framed, in the COVID-19 Conversations vaccine webinar, that “the proof is in the pudding, and the pudding is a phase III clinical trial. . .”. When the term ‘gold standard’ is mentioned with clinical testing, it means a placebo-controlled, double-blind, randomized clinical trial (RCT).7 These large cohort, diverse recruitment, multi-site trials are the most effective method to discern how drug or vaccine candidates perform in human populations. Phase III vaccine trials primarily look for the percentage reduction in incidence (of disease or infection) amongst those vaccinated.7 This takes time for a number of reasons. One is that the disease in question must still be circulating at a volume where data can be collected on a vaccine’s effectiveness. If the disease is mitigated or eradicated through other measures such as effective treatments or non-pharmaceutical interventions like physical distancing, then there is no sick population for comparison. Coupled with the fact that vaccines are a preventative intervention — meaning that they will be administered to healthy people rather than sick ones — the stakes are high to ensure that people are not falling ill or being introduced to new complications. It takes time to flush out these factors.
A way to adapt the phase III playbook to face COVID-19 is to stand up novel, harmonized clinical trial processes that allow health systems, institutional review boards, pharmaceutical company sponsors, third-party contract organizations, and the federal government to collaborate in real-time, sharing data centrally. Dr. John Mascola, Director of the Dale and Betty Bumpers Vaccine Research Center at the National Institute of Allergy and Infectious Diseases, highlighted in the COVID-19 Conversations vaccine webinar that these approaches are assisted by collaborations such as Accelerating COVID-19 Therapeutic Interventions and Vaccines (ACTIV). This is a novel public-private partnership; a consortium of academic, philanthropic, private, and government entities working to ensure innovation is coupled with appropriate scaling so that there is no gap between potential efficacy and availability, when a vaccine or treatment shows promise. This means manufacturing capabilities must be readied before the FDA determines which candidates will meet its stringent requirements for safety and efficacy. The image below is a representation of some ways that public-private collaboration is making the entire drug development road map more cohesive and time-sensitive to achieve these goals.