AD-ding Value: The Science Behind Adenovirus Vector Vaccines
Previously, Research!America published a blog post on the science behind mRNA vaccines. With the approval of a third COVID-19 vaccine in the U.S., this one using a different vaccine technology, this blog post will share the science of adenovirus vector vaccines.
How does the J&J vaccine work? Is it similar to the Pfizer/BioNTech and Moderna vaccines?
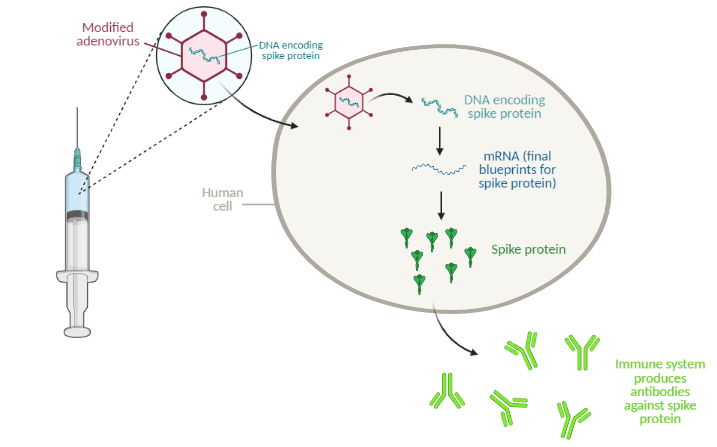
The Johnson & Johnson vaccine is an “adenovirus vector” vaccine. Similar to the mRNA vaccines from Pfizer/BioNTech and Moderna, this type of vaccine uses a snippet of genetic material to elicit an immune response, but it uses a different delivery method. Adenovirus vector vaccines use modified cold viruses to deliver genetic material to the human body. For the COVID-19 vaccine, the adenovirus carries SARS-CoV-2 genes with instructions to make spike protein, a protein found on the surface of SARS-CoV-2.
The adenovirus, carrying DNA instructions for the target protein (in this case, the SARS-CoV-2 spike protein), enters human cells and releases the DNA. From this DNA, the cell makes mRNA, the final blueprints for the protein. The mRNA is then “translated” into protein. The human immune system recognizes the protein as an invader and gets to work generating neutralizing antibodies. The next time the body encounters that same invader (for example, from exposure to the virus), the immune system will quickly get to work neutralizing the threat.
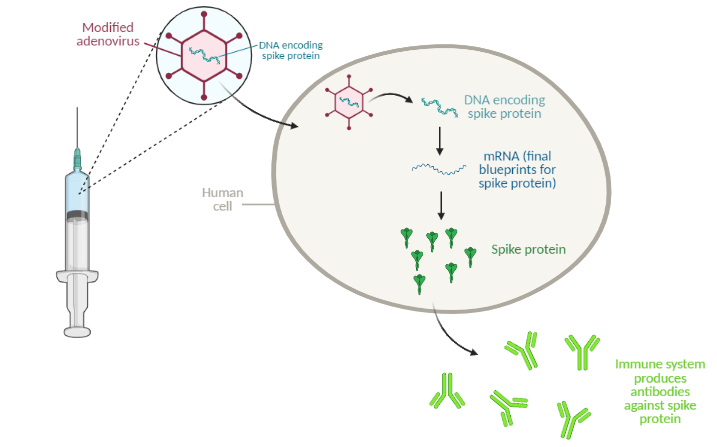
Image made using biorender.com
Is the adenovirus vector vaccine a new technology?
No, but this is the first time such a vaccine will be widely used in humans in the United States. Scientists first started using adenovirus vector technology in laboratory settings in the 1980s. Since then, the technology has become commonplace in labs, and scientists are using it to develop medical applications like gene therapies. Despite their long history, adenovirus vector vaccines are not very common. Before the COVID-19 vaccine was approved, the only adenovirus vaccine routinely used in the U.S. was an oral Rabies vaccine given to wild animals. An Ebola vaccine using the technology was authorized in China during the 2017 Ebola outbreak, and several other adenovirus vaccines, including a flu vaccine, are in development. The Oxford-AstraZeneca COVID-19 vaccine, authorized in the U.K. and other countries, also uses adenovirus vector technology.
Will the adenovirus in vaccines give me a cold?
No. The adenoviruses used in vaccines are different from regular cold viruses in a few important ways. First, they are carefully designed by vaccine developers to be “replication deficient,” meaning they won’t replicate in the body and cause a strong immune response. Second, these viruses are typically based on either more rare adenoviruses or those found in other species, meaning that most people won’t have already been exposed to the virus and the immune system won’t mount an immediate response. The Johnson & Johnson vaccine is based on an adenovirus called Ad26, a less common human adenovirus, and the Oxford-AstraZeneca vaccine is based on a chimpanzee adenovirus.
Can I get COVID-19 from this vaccine?
No. This vaccine contains only a single piece of the SARS-CoV-2 virus, and there would be no way for an active viral infection to take place.
This vaccine is less effective than the mRNA vaccines that have been authorized. Is one type of vaccine better than the other?
From a scientific and epidemiological perspective, each of the three vaccines approved in the U.S. is highly effective and will be an important part of ending the COVID-19 pandemic. The newly-approved adenovirus vector vaccine is approximately 72% effective at preventing symptomatic COVID-19, compared with 95% efficacy for the two authorized mRNA vaccines. For context, however, the flu vaccine is generally 40-60% effective but is still a powerful tool for lessening the severity of flu season. The adenovirus vaccine, while it has a slightly lower overall efficacy, is about 85% effective at preventing severe COVID-19 and virtually 100% effective at preventing hospitalizations from COVID-19.
Are there any advantages of this new vaccine over the mRNA vaccines?
mRNA vaccines require storage at low temperatures – sometimes sub-Arctic temperatures! Clinics and hospitals, especially in more rural locations, often do not have the capacity to store these vaccines. Adenovirus vector vaccines, including the Johnson & Johnson vaccine, can usually be stored at normal refrigerator temperatures. Another advantage of the Johnson & Johnson vaccine is that it requires only one dose, whereas the mRNA vaccines require two doses. This means a batch of the vaccine can stretch further, and it removes the burden of a second appointment.
This blog post was written by Jessica Scott, science communications intern at Research!America. The Science Communications internship is sponsored by the Burroughs Wellcome Fund. She completed her doctoral work in Cell and Molecular Biology at Baylor College of Medicine in 2020.




