Engage and Inform

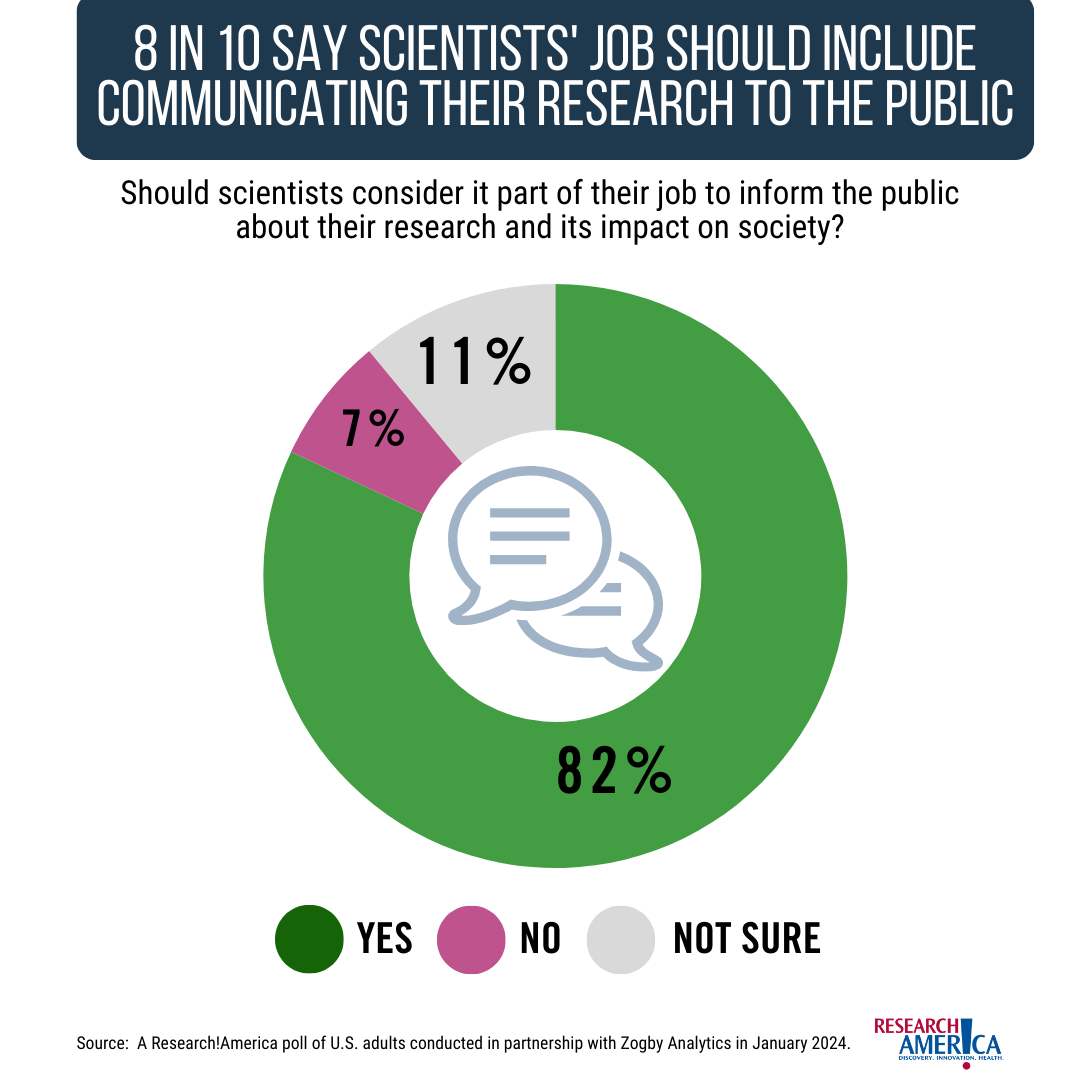
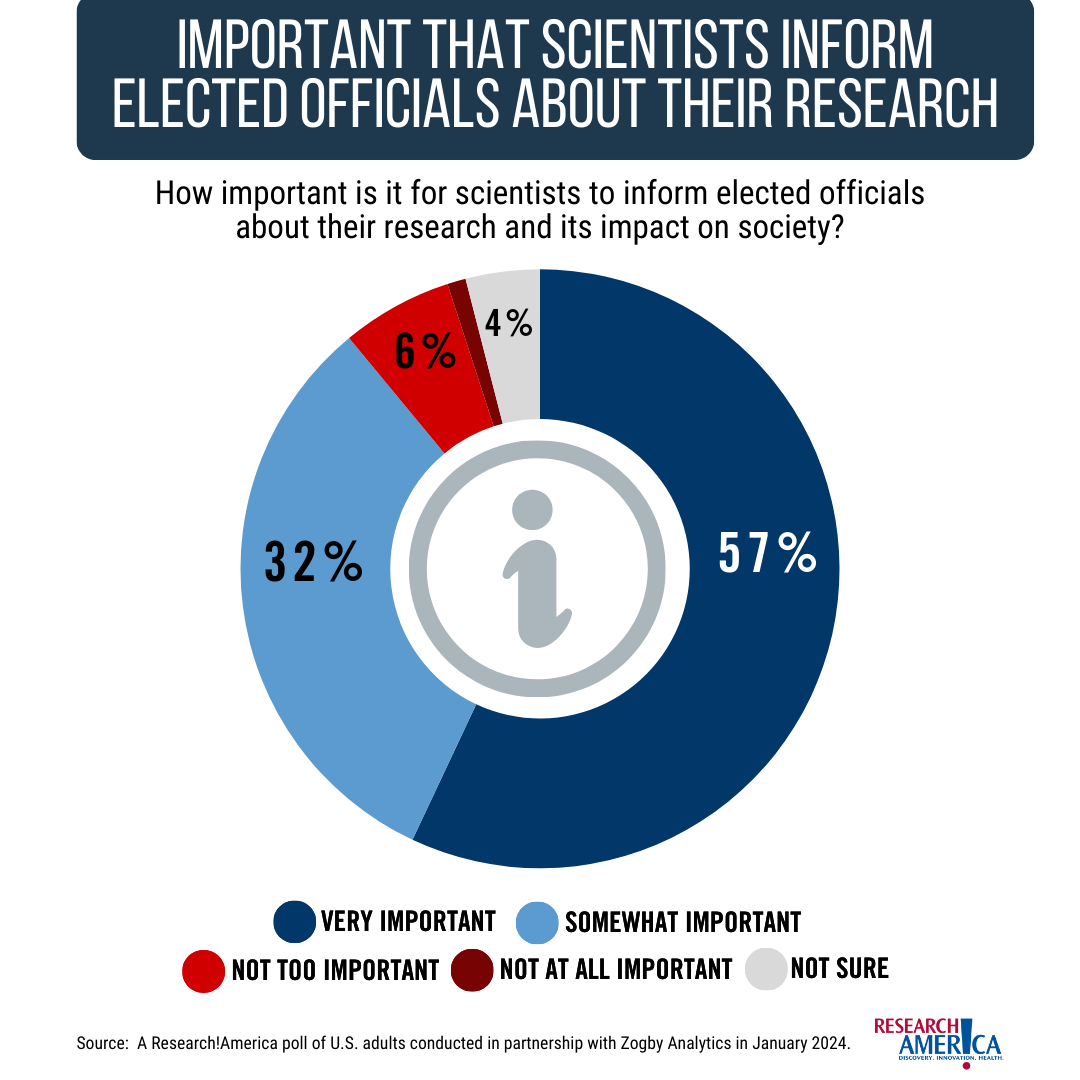
A January 2024 survey commissioned by Research!America included an important finding – that 89% of Americans believe it’s important that scientists inform policymakers about their research and its impact on society. This finding has held constant for the last several years. In the same poll, 82% of Americans surveyed say scientists should consider it part of their job to inform the public about their research.
Among the resources available to help scientists strengthen their communication skills, SciLine, based at the American Association for the Advancement of Science (AAAS), is offering free media training this September for scientists who want to engage with reporters this election season. The virtual, four-hour sessions begin Sept. 5. Space is limited, so be sure to register by Aug. 15. A second organization, the Public Health Communications Collaborative Academy, is offering free communications training for those in public health. Both initiatives will help scientists increase their engagement with their communities!
On the Hill: While Congress is in recess until after Labor Day, there is still plenty of advocacy work to do. Last week we submitted comments responding to Reps. Diana DeGette (D-CO) and Larry Bucshon, MD, (R-IN)’s request for feedback on their “Cures 2.1” initiative. You can read Research!America’s comments here. As a reminder, the 21st Century Cures Act, originally enacted in 2016 and updated with Cures 2.0 in 2021, accelerated medical innovation and approvals by streamlining regulations and enhancing research funding. Another area in which feedback is needed: Aug. 16 is the deadline to submit your organization’s comments to the House Energy and Commerce Committee on Rep. Cathy McMorris Rodgers’s(R-WA) and Rep. Robert Aderholt’s (R-AL) NIH framework proposal.
Sign-on Thank You: As noted in last week’s letter, we’ve drafted a sign-on initiative to Senate Appropriations Committee Chair Patty Murray (D-WA), Subcommittee Chair Susan Collins (R-ME), “Labor-H” Appropriations Subcommittee Chair Tammy Baldwin (D-WI), and Ranking Member Shelly Moore-Capito (R-WV) thanking them for appropriating – on a bipartisan basis – an increase of approximately $2 billion for the NIH in FY25. We welcome organizations and individuals to join this letter by signing on here.
Youth Mental Health: The Centers for Disease Control and Prevention this week released findings from its Youth Risk Behavior Survey, which provides the most updated surveillance data and 10-year trends in high school student health behaviors and experiences, including mental health. While the data show that far too many high school students experience mental health challenges, with higher rates among female and LGBTQ students, it also found some positive developments. For example, the data found that 57% of high school girls reported extreme depressive symptoms in 2021, but that number dropped slightly to 53% in 2023. It’s too soon to know if those numbers indicate a trend, but, at the very least, the number is moving in the right direction.
K-12 Stem Education: In February, Mary had the honor of addressing a workshop convened by the Government-University-Industry Research Roundtable of the National Academies of Science, Engineering, and Medicine (NASEM) to discuss efforts to boost K-12 STEM curriculum. The NASEM just posted the proceedings from that workshop, including Mary’s remarks addressing a recent survey commissioned by the Science and Technology Action Committee (STAC) of professionals in five workforce sectors. The survey found that a plurality in every workforce sector, including K-12 education, says inadequate K-12 STEM education is the principal obstacle to advancing U.S. S&T. She recently spoke with Scientific American about this very issue, which remains timely. We encourage you to review the STAC survey and action report for additional details.
 Clinical Trials Fact Sheet: During our July 30 clinical trials briefing on Capitol Hill, we distributed a concise clinical trials fact sheet describing the clinical trial process and discussing some key challenges. Please use this resource as you have conversations with the public and policymakers about the importance of clinical trials.
Clinical Trials Fact Sheet: During our July 30 clinical trials briefing on Capitol Hill, we distributed a concise clinical trials fact sheet describing the clinical trial process and discussing some key challenges. Please use this resource as you have conversations with the public and policymakers about the importance of clinical trials.
Improving Diversity in Clinical Trials: Speaking of clinical trials, the FDA is soliciting input on its draft guidance on Diversity Action Plans (DAPs) for clinical trials, mandated in 2022 legislation. The DAPs require that clinical trials for drugs, biologics, and high-risk devices include specific diversity plans incorporating statistics on race, ethnicity, sex, and age to ensure that trial demographics reflect those of the overall population. The goal is to enhance research equity and outcomes.You can read more and submit comments through the Federal Register by Sept. 26.
 National Health Research Forum: We are roughly 40 days from an event you won’t want to miss: the 2024 National Health Research Forum on Sept. 17 and 18. Join us for discussions with top federal officials, research leaders, and media on timely topics such as climate change’s impact on health, artificial intelligence (AI) and drug development, nutrition and health, and navigating comorbidities in mental health. The sessions scheduled for Sept. 17 will be virtual, while the Sept. 18 discussions will be in person on the campus of The George Washington University (GWU), followed by a reception at GWU. It’s free and open to all, so register here for the virtual event, and register here for the in-person event. Mark your calendars now!
National Health Research Forum: We are roughly 40 days from an event you won’t want to miss: the 2024 National Health Research Forum on Sept. 17 and 18. Join us for discussions with top federal officials, research leaders, and media on timely topics such as climate change’s impact on health, artificial intelligence (AI) and drug development, nutrition and health, and navigating comorbidities in mental health. The sessions scheduled for Sept. 17 will be virtual, while the Sept. 18 discussions will be in person on the campus of The George Washington University (GWU), followed by a reception at GWU. It’s free and open to all, so register here for the virtual event, and register here for the in-person event. Mark your calendars now!




